SafeEndo BioStructure MTA
MTA powder & liquid for Vital Pulp Therapy and Endodontics procedures
₹1099.00₹1260.00 (-13%)
Benefits

Brand warranty

Secure payments

upto 7 days returnable

Long expiry

EMI
Description
SafeEndo BioStructure MTA is a specialized dental cement based on Di and Tri-Calcium Silicate and Tricalcium Aluminate, derived from advanced material research in inorganic hydraulic powder technology. MTA is indicated for both vital pulp therapy and Endodontics procedures.
BioStructure MTA is a stainproof, tricalcium silicate-based bioactive cement that can be used universally for vital pulp and other endodontic indications in primary and permanent teeth. It is a bioceramic cement that triggers the healing process. It mixes more smoothly, is easier to dispense and has more stable placement, washout resistance and faster clinical setting.
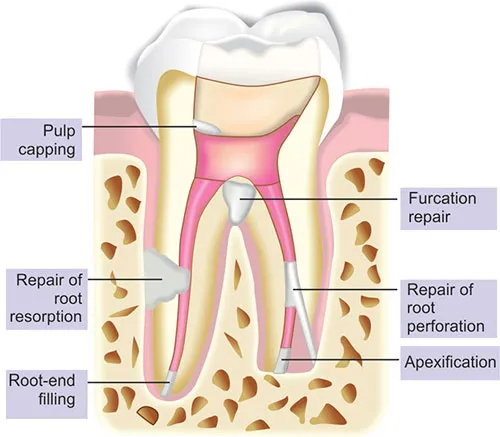
Indications :
- Pulp capping
- Cavity lining
- Pulpotomies
- Root-end fillings
- Apexification
- Apexogenesis
- Perforation repair
- Root resorption
- Obturation (pulpectomy)
Specification
Additional information
| Weight | 0.1 kg |
|---|---|
| Brands |
Features
Features
- Hardens in the presence of moisture
- Controlled setting time
- High mechanical resistance
- Excellent biocompatibility
- Low Solubility
- High alkalinity and microbial activity
Packaging
Packaging
- 1 x Powder 1gm
- 1 x Liquid 2ml
- 1 x Mixing pad
- 1 x Dispensing spoon
Direction to Use
Direction to Use
BioStructure MTA is mixed at room temperature (18-23)°C and humidity (50+10) %. On the block for mixing it is necessary to mix 1 dose (1 g) of the powder with 2 ml of the liquid within 30-40 seconds before receiving thick soft mass.
Pulpotomy
Remove the roof of Pulp Chamber and all remnants of coronal pulp tissue in primary teeth up to bottom of pulp chamber, disinfect and clean it thoroughly with root canal irrigation. Use a small applicator to apply mixed BioStructure MTA material on floor of pulp, remove excess material at the site with a dry cotton pellet, close it with Glass lonomer cement to ensure sealing.
Retrograde filling of Root canal
For retrograde filling of a tooth root apex under anesthesia you should provide access to a tooth root apex (dissect a mucous supra-bony flap), carry out root apex resection and with the help an ultrasonic tip with special diamond hand piece to form a cavity for retrograde filling. After maintenance of hemostasis the cavity in a tooth root apex is filled by the received paste. It is necessary to replace the bone defect with the osteoplastic product, the flap is fixed by the suture.
For Perforation Closure
For closure of the perforation hole into the washed and dried canal you should insert product into a zone of defect, seal and check correctness of its inserting using X-ray. Then the rest canals you should obdurate, isolate with the lining product and restore the tooth crown.
For Root Resorption
For sealing a cavity at the resorption of tooth root it is necessary to provide the access to a zone of the resorption and to carry out tool processing. Then place a product received by mixing of a fast-setting powder with the distilled water (X-ray control) in a cavity of the resorption and isolate its surface by glass- ionomer cement.
For Apexification and Apexogenesis
For apexification of a root you should insert the product into the apical zone and seal, using amalgamate plunger and cotton ball or paper points into the prepared canal. The paste can be condensed, using ultrasonic hand piece without water sprinkling, on average capacity. Under the X-ray control it is necessary to check the correctness of the product accommodation, which should remain as a constant component of a seal of the root channel. Then the rest canals you should obdurate, isolate with the lining product and restore the tooth crown.
For Cavity Liner on Recurrent Carious Area
For a pulp covering the prepared cavity should be processed with antiseptic and a small amount of the product is placed on the naked site with the help of the spherical applicator. Then this zone is covered with a temporary product before the following visit. At the positive dynamics in the following visit it is necessary to remove the temporary product, the medical covering should be isolated with the glass- ionomer cement and restoration is finished.











Reviews
There are no reviews yet.